Background
Because liquid chromatography uses columns with a ‘large’ diameters and a small flow, it takes a long time before the mixture is separated and the product can be collected. As we know now, a HPLC can be a possible solution to this problem. In short, a mixture is passed over a stationary phase with a high pressure, which results in some conditions for the stationary phase. Since the migrating components of the mixture each have different polarities and size, they also have different migration speeds.
Set-up
A HPLC system includes next five elements:
1. A liquid supply system: this is a pump that can generate a high pressure, and one or more water reservoirs
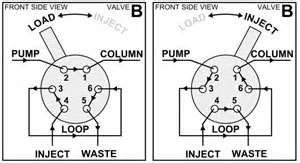
2. An injection system: typically this is a set-up as given in figure 1. This system is made out of stainless steel with different gates and a movable Teflon ring in the middle, which makes it possible to alternately connect two different external gates. In the load position, the mobile phase is loaded on the column by filling the loop with the sample. In the inject position, the sample is dragged to the column.
3. A column: these are mostly made of stainless steel with a more polar mobile phase than stationary phase.
4. A detector: after the mobile phase leaves the column, it passes through a detector for registration of the chromatogram. This detector has a great sensitivity (µg – ng), and there are different ways for detection:
- Ultraviolet-visible light detector, with constant or variable wavelength
- Fluorescence detector
- Breaking detector
- Electrochemical detector
5. A recorder: this is mostly linked to the computer, which analyses and interprets the data immediately
Quantitative analysis
When doing a quantitative analysis, using a HPLC, the method of internal standards is often used. This means that a known amount of a certain substance (internal standard) is added to the calibration sample and the sample that needs to be analyzed. The internal standard undergoes the same processes as the sample, it is injected in the same way and is similarly chromatographed. Because of this, next problems can be detected and registered:
- Inaccuracies in the making of the standard solutions
- Variations in injection volume or temperature of the column
- Drift of the detector signal for both the component that needs to be determined, as the internal standard
A correction for these problems can be done by determining the ratio of the signal of the component that needs to be analyzed and the internal standard. The certain substance, which is the internal standard, must meet certain conditions
- The registered signal and the component to be analyzed should be approximately the same size
- The behavior and detection must coincide with those of the component to be determined without interference from the conditions occurs. Thus, the retention time (tr) of the internal standard is not allowed to vary too much, without, however, overlapping those of the component
To do a quantitative analysis, a calibration curve is prepared, with on the X-axis: the concentration of the component to be determined, and on the Y-axis: the ratio of the component signals of an internal standard. The signals form peaks, wherefrom the surface area and height can be calculated. If the concentration of the internal standard is known exactly, next calculation are possible:
Qc / Qi = (Ac ⁄ As,c ) / (Ai ⁄ As,i ) = As,i / As,c * Ac / Ai
With:
- Qc and Qi : the concentration of the component to be analyzed and internal standard respectively
- Ac and Ai : the peak area of the component to be analyzed and internal standard respectively
- As,c and As,i : the specific surface area of the component to be analyzed and internal standard respectively (specific surface area = area per concentration of the injected material)
For a certain combination of the component to be analyzed and internal standard
As, i / As, c = constant = b
Which results in: Qc / Qi = b * Ac / Ai
Which is the equation of a straight line, and because the concentration of the internal standard is constant for every sample (Qi = constant), it can be concluded that:
Ac / Ai ~ Qc
If the basis of the registered peaks are constant, the peak area is directly proportional to the peak height which means that:
Hc / Hi ~ Qc
With Hc and Hi : peak height of the component to be analyzed and internal standard respectively.
This means that it is not necessary to know the concentration of the internal standard exactly, as long as the added amount thereof is always exactly the same.